Trade secrets v. patents – conflict of IP protection with AI in healthcare wearable devices
Digital technology and AI have hijacked all the industries, and the healthcare sector is not an exception. AI has helped in the evolution of the digital healthcare and pharmaceutical industry. This has expanded the scope of using AI in managing and protecting innovation but also raised concerns on many IP issues. The importance of trade secrets in organisations increases with the adoption of new technologies. The debate over whether intellectual property protection in the field of AI should be implemented within the framework of patent protection or as a trade secret has become an endless discussion.
Wearable Devices and Software as Medical Devices (SaMD)
Wearable devices are different kinds of electrical devices that can be worn externally in parts of the body. Fitness tracker, hearing aid devices, and smart watches are some of the common examples of wearable devices. SaMD’s are the software technologies that are embedded in the advanced wearable devices to track or perform activities that are more than general fitness tracking. Advanced smart watches, smart rings, wearable ECG monitors and mental health applications are some of the common examples of the devices that uses SaMD to operate.
Advanced Smart Watch – Use of SaMD
Wearable devices such as a smart watch are an example of SaMD. Initially smart watches were used for tracking the number of steps only. However, with enhanced features they can also give results on the heart rate, skin temperature, predict menstrual cycles, ECG and many more such activities. The interpretation of FDA, Section 201(h) emphasises on the regulatory requirements of wearable devices.[1] Section 201(h) of FDA clarifies that the definition of devices includes devices that are used for the purpose to diagnoses, mitigation, treatment or prevention of diseases. Smart watches can be categorised under the bracket of “diagnoses” if the data collected by the watch is used for diagnosing and medical care for a cardiovascular disease. Similarly, “mitigation” and “prevention of disease” is possible by monitoring the data on regular intervals to supervise the heart rate and ECG thereby also assisting doctors during treatment. Hence, advanced smart watches that are utilised for the services as covered under the Section 201(h) could help healthcare professionals to connect the dots between In Vivo and In Vitro diagnostic.
SaMD on smart watches and related similar devices require special attention as they include personal sensitive data or personally identifiable information that can be utilised during clinical trials to collect data from the participants. The IP strategy best suited for these wearable devices has become a topic of discussion as patents and trade secrets both play a crucial role in protecting the proprietary information and inventions of an organisation. US laws and European Directives provide protection to trade secrets and patents relating to different parameters of IP. The European AI Act has also added complex layer on organisations to ascertain their IP strategies.
Role of Patents v. Role of Tade Secrets
Patents protect inventions and generate revenue for an organisation through licenses, on another hand trade secrets preserve the confidentiality of a process and novelty of an invention. Hence, both are important aspects in protecting innovations of an organisation. The use of AI in healthcare devices complicates the correct IP protection due to the basic characteristics of AI as a technology. After a SaMD is deployed in a wearable device it has the potential to learn from the data that is collected from an individual wearing the device. Although, protecting the SaMD via a patent can protect the software and the wearable device, but challenges the scope and specification that could be granted as a patent as opposed to a trade secret.
The table below shows some of the laws that are applicable to wearable devices:
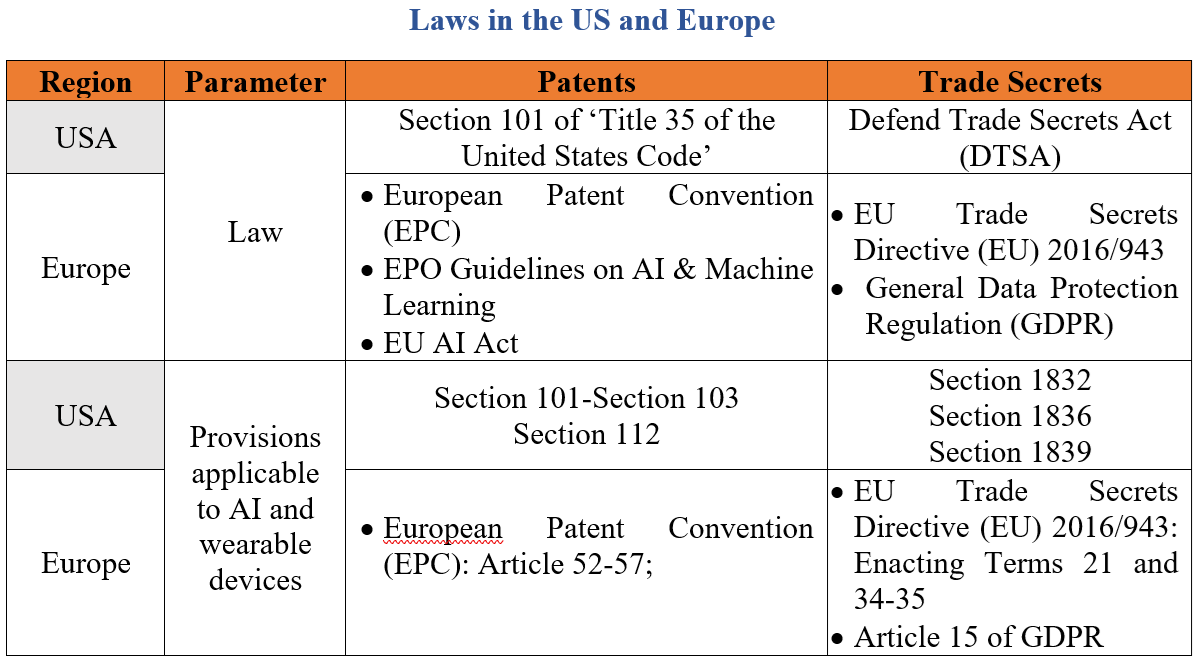
Hence, the above table provides an understanding on the regulatory frameworks of Europe and the USA applicable to wearable devices. It is important to note that wearable devices provide enormous benefits by providing aids such as radiological imaging, clinical diagnosis and drug discovery which are the result of raw data and evidence generated from the data collected to services such as customised fitness programmes, user guides and trainings.[2]
Role of Patents: Alice Test
The patentability of any SaMD is a complex question across jurisdictions. The Alice Corp. v CLS Bank International[3] case law in the US opened the doors for the patent applications on software patents thereby broadening the interpretation of the subject matter of patents and facilitating the promotion of innovation under Section 101 of Title 35 of the United States Code. This test determines the interpretation of software to be covered in the ambit of patent and is known as “Alice Test”. This test provides a regulatory framework for preserving innovation and novelty of advanced technologies.
The two-step process involved in Alice Test consists of the following:
- Identifying if the patent claim is an abstract idea as defined by the United States Patent and Trademark Office (USPTO). According to the USPTO, abstract ideas are mathematical concepts or mental processes.
- Once the identification of the patent claim is completed, it is determined if the patent claim is an abstract idea with a practical application. In instances of meeting the criteria of practical application, the claim is eligible for patenting. If the claim does fall under the concept of an abstract idea, it undergoes further scrutiny.
In the recent US complaint filed by CardiacSense LTD against Casio Computer Company Ltd. for “Willful Patent Infringement” in Texas, CardiacSense alleges that Casio’s G-SHOCK series of multi-sensor sport watches infringe a patent on a personal device for measuring and analysing a user’s training activity.[4] The technology for measuring the physical movement of individuals used in both devices including a combination of sensors, such as accelerometers and gyroscopes, is the technology issue of the complaint. The patent is owned by CardiacSense on a “Training and Instructing Support Device”[5] for providing a comprehensive result on an athlete’s motion thereby producing an accurate result. The Alice test may impact the conclusive outcome on this case law due to the following rationale:
- The Section 101 of ‘Title 35 of the United States Code’ has been the basis to determine the patent eligibility.
- In a similar suit filed by CardiacSense LTD on COROS Wearables Inc[6], a ruling was set in favour of CardiacSense on the grounds as provided below:
a) Alice Test
COROS argument on “monitoring physical activity” as an abstract idea was challenged by CardiacSense on the ground that it was a specific technical solution that improved the prior art with the use of multi-sensor wearable device.
b) Judgement
The Central District of California ruled in favour of CardiacSense stating that patent claims were not abstract and described an inventive step.
To sum up, the judgement on COROS Wearables Inc will also impact the G-SHOCK series case as CardiacSense had substantial business in the Eastern District of Texas (place of filing the claim) on the similar grounds.
Importance of trade secrets
Trade secrets are complex protective rights as compared to patent rights. Patent claim eligibility can be determined with technical information, whereas trade secret protection is determined by protecting the proprietary rights of an organisation. SaMD is a developing technology that may provide many more health benefits to users and help an organisation to become dominant the market. In a competitive industry, it becomes important for organisations to preserve propitiatory information for the following reasons:
- Inventions at an early stage contain data sets that help in building the algorithm of the software. Digital Health Analytics (DHA) provide a skeletal structure to modern organisations which have a mission to create better healthcare. The DHA helps in creating a value chain from early detection to drug discovery. Thus, data sets are a gold mine for any industry that serves the purpose of healthcare.
- SaMD R&D includes raw data and personal identifiable information which can be provided to Clinical Research Organisations (CRO) to check the effectiveness of the technology. SaMD also helps in tracking gynaecological cycles, pregnancy phases and other health aspects of women health. This has led to the evolution of health-tech companies in the untapped space of “Femtech” that is conducting R&D for women’s health diagnostics and early detection of diseases such as ovarian cancer, maternal and foetal health, and self-help breast examination with the help of data incorporated by the users in applications.[7] Therefore, data protection regulations also partially cover aspects on trade secrets.
- SaMD is a digital object with an intangible existence and Personal Identifiable Information (PII). As all the organisations function in a matrixed structure, data transfer across the globe is common. This necessitates that the data must be anonymised for authorised sharing of PII to any third-party intermediary and ensuring that the rationale of collecting the raw data is protected by trade secrets.
To conclude, healthcare wearable devices are a development in the health tech sector that has revolutionised the industry through the use of AI. This has introduced new IP challenges and has shown the importance of incorporating IP strategies into business models.
 About the author:
About the author:
Trishala is a Corporate Counsel at Thermo Fisher Scientific, India. She has completed a postgraduate degree in IT and IP law from the University of East Anglia, Norwich, UK. Her areas of personal interest are analysing inter-disciplinary subject matters related to IP and developing technologies such as blockchain and AI.
The assumptions, views and opinions expressed in this article are those of the author in her personal capacity and do not in any manner reflect or represent any policy, position, opinion, view or idea of Thermo Fisher Scientific, India.
________________________________
[1] Briana Mittleman, ‘FDA pathway for wearable medical devices’, Standford Law School Blogs, FDA pathway for wearable medical devices – Law and Biosciences Blog – Stanford Law School, accessed 9 March 2025.
[2] Aseet Patel and Michelle Yongmei Zhang, ‘Patents and Trade Secrets: IP Protection of AI in Digital Health and Wearable Devices’, American Health Law Association, AHLA – Patents and Trade Secrets: IP Protection of AI in Digital Health and Wearable Devices, accessed on 6 May 2025.
[3] 573 U.S. 208 Alice Corp. v. CLS Bank International (2014)
[4] Cardiacsense Ltd v. Casio Computer Co Ltd, 2:25-cv-00674, E.D. Tex., 07/02/2025
[5] Cardiacsense Ltd., Training and Instructing Support Device, USA 7980998, July 19, 2011
[6] CardiacSense Ltd v Coros Wearables Inc, No 6:24-cv-00280 (WD Tex, filed 22 May 2024, closed 19 August 2024)
[7] Anonymus, “20 Best Femtech Companies Empowering Women’s Health and Wellness” (DOIT Software, 30 July 2025) <Top 20 Femtech Companies: Pioneers in Women’s Health > accessed 1 October 2025



