The role of IP in the digital transformation of the MedTech industry: Case study B. Braun
The MedTech industry has experienced impressive growth over the last decades and the market is still growing enormously. IP is one of the key drivers for successful digital transformation within the healthcare industry. By taking a comprehensive approach to digitalization and IP management, B. Braun can deliver products and services more quickly, boost innovation in the industry and hold down costs. This case study shows how IP design is used to improve innovativeness in a competitive environment and act successfully in growing and digitally transforming markets.
MedTech companies are currently affected and challenged by three concurrent technology-driven shifts:
- Increasing pace of digitalization: Posing questions regarding ecosystem positioning, digital excellence, and data monetization, from machine automation to connectivity and analytics, from single machines to entire value networks.
- Shift to value-based healthcare: Posing questions regarding pricing models, customer and patient relationships, from supplier to ecosystem partner, from hardware to software and data-driven digital services.
- New foundation of digital technologies: Posing questions regarding value creation, product and service innovation, and required capabilities, from delivering spare parts to leveraging 3-D printing, from human experts to self-learning systems.
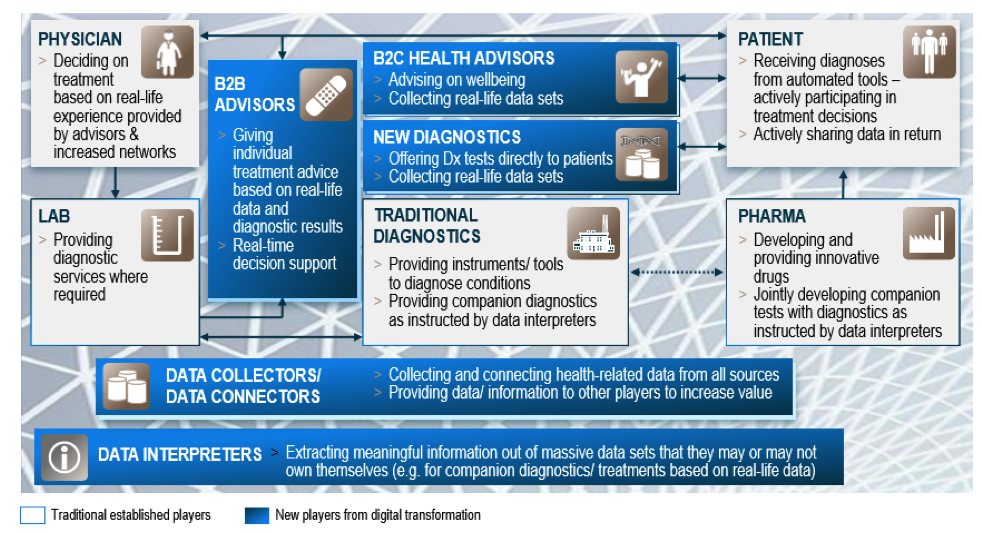
Digitization offers the prerequisite for future growth and is therefore the topic of the day for companies to remain competitive. But how can digitization deliver value and growth? The answer: By using digital technologies to improve operational excellence, reduce costs, enable new business models and generate new revenue opportunities. Few industries are as heavily regulated as the
pharmaceutical and healthcare industries. Companies must prove that their products are developed and produced according to structured, repeatable and controlled processes. Despite these strict requirements, the market volume and technological possibilities are attracting more and more technology giants that have their roots in the consumer environment.
Cannot open the video? Please click: https://youtu.be/O5DNnPKpgJY
For example, with the new generation of Apple Watch and the ECG integrated into it, Apple has for the first time approved a medical product in the USA. Google Fit also promises support for a healthier and more active life. Innovations in products and new
business models will continue to be a key success factor in the future, especially for established companies – for example, to bring personalized medical products up to batch size 1 onto the market cost-efficiently.
Google appears to have plans to develop its own electronic record (HER) for clinicians that gathers patients’ medical records and then leverages machine learning to predict clinical outcomes, according to patent applications. Just around the ECG App, iDevice User Interface for Health Monitoring, Apple filed 56 patent applications.
The digitization of products and processes, however, has dramatically changed the game for everyone. Consumers’ expectations about healthcare services are increasingly being informed by their experiences with large digital-born companies. With this “customer experience” frame in mind, healthcare companies are seeking to integrate the latest technologies into existing business models and IT architectures to improve services. At the same time, they are grappling with new, nontraditional entrants to the marketplace (IBM, and Microsoft, for instance), as well as ever-present regulatory and risk-related concerns. More and more healthcare companies worldwide are finding that digital technologies must be managed not as utilities but as strategic assets. Some are attempting to bridge the gap between legacy and digital IT by undertaking complex systems transformations. One large healthcare-technology company is experimenting with ways to maintain its existing IT architecture while using analytics to securely mine the data it collects for useful business insights. Similarly, a large drug maker is exploring the use of cloud platforms to reduce data storage and processing costs and to boost the speed of its R&D efforts.
Still, most pharmaceutical and medical-technology companies are digital laggards compared with companies in travel, retail, telecommunications, and other sectors. Their digital-transformation efforts can stall for many of the same reasons such efforts are thwarted in other sectors—for instance, a limited understanding of the specific ways that implementation of new technologies across complex product and services lines can create business value, a shortage of native digital talent, and insufficient focus on digital topics from senior leadership.
Cannot open the video? Please click: https://youtu.be/vcvIQ-NDuKE
Braun is confronted with the digital transformation of the healthcare ecosystem. In addition to high complexity, this ecosystem is also characterized by a vast number of regulatory requirements. These requirements deeply affect the processes of MedTech companies, their products, innovation, and technology, including times to market. B. Braun stands out in terms of innovation competence by not just offering isolated products, but by understanding the sometimes highly complex processes of its customers, and offering holistic, integrative solutions and benefits. The Advanced IP Management initiative has strengthened B. Braun’s ability to better capture and derive exclusivities from its actual innovation performance by means of IP, and to better support the increasingly digital approaches, including the user perspective.
Authors of the case study:
 Dr. Hans-Otto Maier
Dr. Hans-Otto Maier
Dr. Maier is Senior Vice President Innovation & IP-Management Divisions Hospital Care and Out Patient Market at B. Braun Melsungen AG – one of the world´s leading healthcare suppliers. In his 40 years career in the medical and pharma industry he gained comprehensive experiences not only in the development of medical devices and pharmaceutical products but also in marketing as well as in production. With this knowledge, he and his team has the goal to strengthen the innovation leadership of B. Braun in the mentioned fields.
Another area of responsibility is the management of the company Nutrichem diät + pharma GmbH. This firm develops and produces enteral nutrition, nutritional supplements and sports nutrition.
Within the DIN Standards Committee Medicine (NAMed) Dr. Maier is Chairman of the Committee NA 063-01-02 AA Catheter, Drainage. He is also lecturer at the University of Applied Science Mittelhessen and the University Furtwangen.
 Solvej Fritsche
Solvej Fritsche
Mrs. Solvej Fritsche, Director IP Melsungen at B. Braun Melsungen AG, is specialized in IP management, patent disputes and employee invention law. Mrs. Fritsche is a graduated engineer in medical technology and made her first experiences amongst others in the automotive supply industry. In 2008 she started as patent engineer in a patent law firm and in 2011 she was admitted as European Patent Attorney. Since 2014 she is Director of IP Melsungen at B. Braun.
 Prof. Dr. Alexander Wurzer
Prof. Dr. Alexander Wurzer
Dr. Wurzer is Adjunct Professor for IP Management at the Center for International Intellectual Property Studies (Centre d’Etudes Internationales de la Propriété Industrielle, CEIPI) at the University of Strasbourg, where he has been Director of Studies for the Master’s degree in Intellectual Property Law and Management (MIPLM) since 2007. Prof. Dr. Wurzer is Director of the Steinbeis Transfer Institute for Intellectual Property Management at Steinbeis University Berlin. He is Managing Partner at WURZER & KOLLEGEN GmbH, a consulting firm specializing in strategic IP management. Prof. Dr. Wurzer is Chairman of DIN committees DIN 77006 for quality in IP management and DIN 77100 for patent valuation. He is a member of the Board of Directors of “Deutsches Institut für Erfindungswesen e.V.” (D.I.E.), Spokesman of the Board of Trustees awarding the Diesel Medal, and Fellow at the Alta Scuola Politecnica at Milan/Turin Polytechnic. He was also a jury member for the 2018 German Innovation Award of the German Design Council and is a member of the group of experts of the European Commission.



